1224
Views & Citations224
Likes & Shares
Vaccines
against amyloid-beta-peptide (Aβ) have been widely investigated as a potential
immunotherapeutic approach for Alzheimer´s disease (AD) during the past years.
However, previous vaccines tested in mice and humans have failed to address the
major hallmarks of AD, such as senile plaque-like Aβ deposits,
plaque-associated dystrophic neurites and glial proinflammatory cytokines, due
to massive T-cell activation that resulted in a meningoencephalitis-like
reaction. We have recently developed a vaccine (EB101), based on passive
immunotherapy, that delivered Aβ1-42 in a novel immunogen-adjuvant consisting
of sphingosine-1-phosphate (S1P)-containing niosomes, to APP/PS1 transgenic
mice before and after detectable AD-like pathological effects. Quantitative
analysis of amyloid burden, performed in the affected brain regions, showed a
notable decrease in the mean area of Aβ plaques when EB101 was compared with
other treatments, demonstrating that S1P plays a key role as a
regenerative-neuroprotective agent during the development and consolidation
periods of AD neuropathology. In the present study, we screened the efficacy of
each component of our immunogenic vaccine EB101 in the APP/PS1 transgenic mouse
model, in order to characterize the positive effect of S1P in the
immunotherapeutic response. Complementary results demonstrated that EB101
vaccine significantly prevents and reverses the AD neuropathology hallmarks by clearing
Aβ plaques, reducing dystrophic plaque neurites and minimizing
neuroinflammation, while markedly reducing neuronal degeneration and thus
prolonging lifespan in the APP/PS1 transgenic mouse model, paving the way for
future relevant clinical trials.
Keywords: Alzheimer’s disease, Amyloid beta peptide,
APP/PS1 transgenic mice, Immunotherapy, Vaccine
Abbreviations: AD: Alzheimer’s Disease; Aβ: β-Amyloid Protein; APP: Amyloid Precursor Protein; CA1: Field CA1 Hippocampus; CA3: Field CA3 Hippocampus; Cx: Cortex; DG: Dentate Gyrus; Ent: Entorhinal
Cortex; GrDG: Granular Dentate
Gyrus; IL: Interleukin; MODG: Molecular Dentate Gyrus; PoDG: Polymorph Dentate Gyrus; Tg: Transgenic; Th: T Helper.
INTRODUCTION
In
Western countries, Alzheimer’s disease (AD) is the most frequent form of
dementia, affecting up to one-third of elderly individuals [1]. Its
pathological hallmarks in the brain are characterized by the accumulation of
amyloid-β (Aβ) peptide amyloid plaques, neurofibrillary tangles with
hyperphosphorylated tau proteins, neuronal-synaptic loss affecting particularly
the neocortex, hippocampus and entorhinal regions [2]. Taking advantage of the
potential aspects of the APP/PS1 double-transgenic mouse line, numerous
investigations have used this particular model to study emergent therapies to
prevent and reduce the neurodegenerative features of AD.
The purpose of the present study is to investigate the efficacy of each component of our immunogenic vaccine
EB101 in the APP/PS1 transgenic mouse model, in order to characterize the
positive effect of S1P in the immunotherapeutic response of AD-like pathology.
This goal is intended to be achieved by neurohistochemical methods that will
analyze the functionality of a new physiological adjuvant design, composed of
naturally-occurring phospholipids that prove safe and efficacious in other
types of vaccines (e.g. influenza), with an added biologically active
phospholipid (S1P), known to stimulate an anti-inflammatory reaction and to act
as a neuronal regenerating agent in vitro and in vivo studies [3]. Based on our
previous studies, the preventive and therapeutic effect of the EB101 vaccine in
APP/PS1 mice, designed to address the pitfalls of previous vaccines, conserve
the immunogenicity targeted for the reduction of Aβ burden deposits, but avoid
the massive activation of T-cell-mediated immune response that potentially
causes adverse inflammatory effects. Moreover, this cost-effective vaccine was
formulated to reduce Aβ burden by generating a robust anti-Aβ antibody
production, in order to prevent or slow down the main AD-like pathological
alterations and stimulating an anti-inflammatory T-helper (Th) 2 immune
response.
MATERIALS
AND METHODS
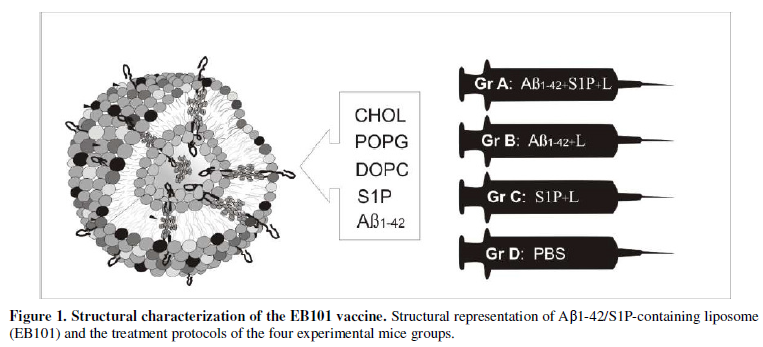
Experimental design
Two experimental treatment studies were carried out; one before AD
onset, starting at 7 weeks of age (preventive treatment), and the other after
AD onset at 35 weeks of age, when neuropathological AD characteristics were
well established (therapeutic treatment). APPswe/PS1dE9 double-transgenic mice
(B6C3F1/J), expressing a chimeric mouse/human amyloid precursor protein
(Mo/HuAPP695swe) and human presenilin 1 (PS1-dE9) mutants, were randomly
divided into these two experimental studies, and in each study they were
divided into four treatment groups (Figure
1), as follows: Preventive treatment: Group A was formed by 16 mice (12
transgenic and 4 wild-type mice; 12+4) that were immunized with a cocktail of
synthetic human Aβ42/S1P-containing niosomes (EB101); group B, formed by 16
mice (12+4), immunized with Aβ42/liposome without S1P; group C, formed by 16
mice (12+4) immunized with S1P-containing niosomes; and group D, formed by 10
mice (6 transgenic and 4 wild-type mice, control) inoculated with PBS.
Therapeutic treatment: The same treatments were administrated in groups A, B
and C, formed by 16 mice in each group, while group D, formed by 10 mice
inoculated with PBS, was the control. Mice were immunized with nine injections
for seven months, inoculating 100µl containing a cocktail of Aβ (100µg) and
phospholipid mix (1mg) per injection. A representation of the model is
portrayed in Figure 1.
Experimental procedure
Immunization
procedures: 44 APPswe/PS1dE9 transgenic mice
were inoculated intraperitoneally with 100 μl per injection of EB101 (group A),
only liposome complex EB102 (group B) or PBS (group C), during seven months (9
injections) for each treatment period. The immunization protocol was
systematically used in previous similar studies [4,5,6] and was followed
strictly, with 3 injections every two weeks in the first month, and then one
injection each following month. This immunization regimen was followed in the
preventive and therapeutical periods.
Immunohistochemistry and
imaging: Immunohistochemical analysis and imaging were prepared
as previously reported [5,6].
Statistical analyses. All statistical parameters were
performed with the use of SPSS (version 11.0; SPSS Inc, Chicago) and a P value
<0.05 indicated statistical significance. The average range of Aβ plaque
density and burden area were analyzed by using a two-factor repeated-measures
analysis of variance (ANOVA) followed by a post hoc analysis when relevant. All
data were expressed as the mean ± SEM.
EB101 vaccine prevents and reverse AD neuropathology as we recently
demonstrated, by the marked reduction of Aβ plaques, plaque-associated dystrophic
neurites and astrocytosis in affected mouse brain areas. Next, we conducted an
EB101 component screening study in order to elucidate the effective role of
each in the prevention of Aβ deposits in the APP/PS1 mouse brain. Aβ plaques
and vascular amyloid loads were detected, counted and measured as the
percentage of total surface stained by monoclonal anti-Aβ antibody in the
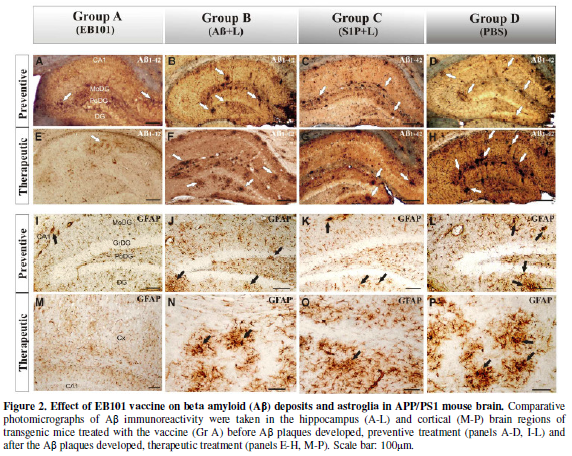
hippocampal and cortical sections. At the first experimental phase (preventive
treatment), the amyloid plaque load observed in the representative
photomicrographs (Figure 2) showed a
significant difference across the four experimental groups (ANOVA, F=3.15,
P<0.001).
In this experiment we observed that EB101-immunized mice (Group A, Figure 2A) showed a
significantly reduced hippocampal load when compared with the different vaccine
components (Groups B-C, Figure 2B, 2C).
In fact, the Aβ
load in these different vaccine component groups (B-C) was not
significantly different from PBS-treated mice (Group D, Figure 2D), although there was a correlation between the
presence of S1P+Aβ in treatment regime and the total Aβ burden. The total Aβ
plaque density and size quantification also supported this data, being
performed by image analysis software of anti-Aβ antibody-stained sections in
the hippocampus (Figure 2A-2D).
Statistical analysis of the data obtained in the present study demonstrates
that the mean Aβ plaque burden (Figure
2A) of group A (25 ± 5 p/s) was significantly different from the other
treated groups (63 ± 6 p/s in 2B; 65 ± 6 p/s in 2C; 68 ± 6 p/s in 2D), which
represents a notable decrease in the mean percentage of Aβ plaque area of
30.1-38.2% in the EB101-immunized mouse group relative to other tested groups.
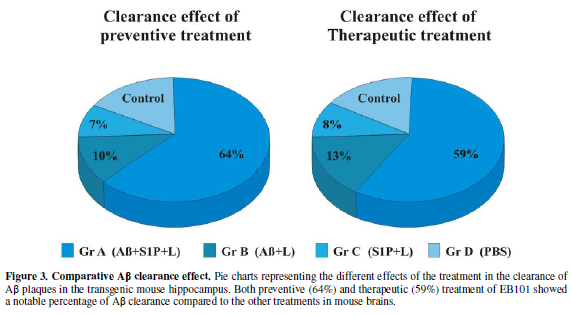
Therefore, these data show that in the preventive treatment period (Figure 3), the clearance effect of the
EB101 vaccine on Aβ plaques per section was about 64% (Figure 2A, Figure 3) compared to positive controls, while 10% was
detected in Aβ42/Liposome without S1P (B) and 7% in Liposome with S1P-treated
mice (C).
Efficacy of S1P-containing niosomes (EB101)
in clearing Aβ plaque burden
At the second experimental phase, (therapeutic treatment), we compared
the effect of EB101 vaccine with different vaccine component groups in the
reduction of Aβ in the brain after the plaques were established in the
35-week-old APP/PS1 transgenic mice (Figure
2E-2H). The results obtained showed that Aβ deposits were significantly
reduced in the hippocampal brain sections of the EB101-treated mice (A),
markedly different from the Aβ burden density observed in the different vaccine
component groups (B-C). Photomicrographs
of EB101-treated mice showed a few compacted Aβ plaques located at the CA1
layer of the hippocampus (Figure 2E),
while in the other experimental groups no reduction in plaque density was
observed (Figure 2F-2H), numerous
large compacted Aβ plaques being located in almost every layer of each brain
section. The data obtained showed that the treatment groups without Aβ1-42, C (Figure 2G) and D (Figure 2H), were the section most densely populated with Aβ
plaques, while a slight reduction in this hallmark was observed in mouse group
B (Figure 2F). As observed in the
preventive treatment, the mean burden of Aβ plaques in group A (35 ± 4; Figure 3) was significantly different
from the other treated groups (65 ± 5 in B; 70 ± 5 in C; 80 ± 6 in D), while
the Aβ plaque area in the EB101-immunized mouse group was reduced by
29.7-34.5%, relative to the other groups tested (Figure 3). In both preventive and therapeutic treatments, a reduced
Aβ plaque staining intensity was detected in the EB101-immunized mouse group,
as shown in the photomicrographs of figure
2A,2E, contrasting with the intense Aβ-immunoreactivity of Aβ plaques in
the other experimental groups.
Immune response
effects of S1P-containing niosomes (EB101) in APP/PS1 mouse models
In order to analyze astrocyte activation in the hippocampal regions of
APP/PS1 mice, which is known to be implicated in neuroinflammation,
amyloidogenesis and neuronal cell death in AD, we used immunohistochemical
analysis to quantify and compare the GFAP-reactive cells in the affected
regions after each treatment period (Figure
2I-P). At the end of the preventive treatment (Figure 2I-L), EB101 significantly reduced the density of
GFAP-reactive cell clusters in the hippocampal and cortical sections as
compared with other treatments, such as Aβ/Lip without S1P (B), Lip with S1P
(C) and PBS (D). Only a few scattered GFAP-reactive clusters, mainly at the CA1
hippocampal layers, were observed in the transverse section of the mouse brains
treated with EB101 (Figure 2I),
markedly contrasting with the numerous dystrophic reactive astrocytes observed
in different hippocampal areas of mouse brains in groups B-D (Figure 2J-2L). After therapeutic
treatment, mouse brains treated with EB101 were similar to those of control
mice, mostly devoid of reactive GFAP clusters, except a few scattered ones in
the CA1 hippocampal layers (Figure 2M).
In contrast, there was a moderate density in group B (Figure 2N) and extensive density in group C (Figure 2O), and group D mice (Figure
2P). No astrocytosis was observed in control mice of each group during
preventive or therapeutic treatment periods.
DISCUSSION
Liposome-based vaccines have demonstrated great effective potential in
preventing and treating AD [7]. Based on previous effectiveness of active
immunotherapy in reducing Aβ plaque accumulation [8], we have developed a new
vaccine cocktail to circumvent the previous vaccine failures, due to an
extensive T-cell-mediated immune response [9,10], by judiciously selecting an
adjuvant that addresses all the above targets while avoiding the massive
autoimmune activation of T-cells. This novel immunogen-adjuvant configuration
consists of a physiological matrix, niosomes composed of naturally-occurring
phospholipids (phosphatidylglycerol, phosphatidylcholine, and cholesterol),
that has proven to be safe and efficacious in other types of vaccines. In order
to increase the immunogenic potential we added a biologically active
sphingolipid, sphingosine-1-phosphate (S1P) to this phospholipidic
configuration. S1P is a phosphorylated product of sphingosine, catalyzed by
sphingosine kinase, which has been reported to be an important lipid mediator
acting both inside and outside the cell membrane [11,12]. Due to its role in
the regulation of neuronal function, S1P facilitates glutamate secretion in
hippocampal neurons by secretion actions, being involved in regulatory mechanisms
of synaptic transmission. In particular, S1P itself causes glutamate secretion
from presynaptic sites and potentiates glutamate-induced transmitter secretion
in primary hippocampal neurons [13], possibly facilitating the formation of a
positive activation cycle in excitatory neurons such as glutaminergic neurons.
As extracellular effects, S1P binds to members of GTP-binding protein
(G-protein)-coupled S1P receptor family (S1P1-5), triggering active immunity,
angiogenesis, cell motility, and neurite growth [14,15]. As intracellular
effects, S1P modulates cellular calcium mobilization, cell growth, and the
inhibition of apoptosis [16,17].
Recently, the potential effect of S1P in neuronal regeneration was
demonstrated in detail, since intracellular S1P enhances nerve growth
factor-induced excitability in the sensory neurons of rats [18,19], controls
migration of neuronal stem cells toward a site of spinal cord injury [20], and
induces cytoskeletal rearrangements through small G-protein Rac activation
[21], which seems to facilitate synaptic vesicle fusion to plasma membranes,
enhancing transmitter secretion. Taking advantage of its important role in
neuronal regeneration, cell growth, suppression of apoptosis and glutamate
secretion from presynaptic sites that are affected in AD neuropathology, we
incorporated the S1P into the phospholipid liposome to form a
phospholipid-S1P-liposome matrix used as an immunogen-adjuvant to deliver the
active antigen, Aβ1-42 [5]. Thus, this combination added a regenerative and anti-inflammatory
component to the vaccine, key elements to increase neuronal activity and
prevent inflammation in the brains of APPswe/PS1dE9 transgenic mice, improving
the immunotherapeutic results previously reported by the studies based on
Aβ-immunization [22,23], while minimizing potential side effects.
EB101 vaccine was generated by using the hydration-rehydration method
to efectively combine phospholipids, S1P and liposomes, forming niosomes
against Aβ1-42 oligomers. This was used in other vaccines for an efficient
liposomal-protein configuration, overcoming the immune pathological response
encountered when using other types of adjuvants such as Freund’s adjuvant
[24,25], Quil-A [26], QS-21 and the detergent polysorbate 80 to solubilize Aβ,
which are believed to induce a proinflammatory Th1 response [10,27]. Over the
last decade, Aβ immunotherapy studies in APP-tg mice have reported different Aβ
antibody levels, depending on the mouse model used, the immunization
methodology, and the type of adjuvant for the vaccine formulation, emphasizing
the significant impact of the adjuvants on the immune response evoked [28].
Considering this, we strategically planned a novel adjuvant-liposomal vaccine
formulation in which the phospholipid-S1P-liposomes represent the pivotal
structure that provides an inmunotherapeutic response markedly different from
previous vaccine formulations tested in AD-like mice. Moreover, the significant
effect of EB101, obtained in mice after the establishment of Aβ plaques in the
hippocampus/neocortex and subsequent neuropathological changes, shows that
EB101 vaccine presents a tremendous potential as a therapeutic agent to reverse
neuropathological hallmarks in the AD-like brain, which represent an effective
immunotherapeutic approach [29,5,6]. The results obtained in the preventive and
therapeutic treatments showed that EB101 vaccine is efficacious not only in
preventing the development of AD-like pathology but also in reducing it once
established. The APPswe/PS1dE9 transgenic mice showed tiny and compact
nonfibrillary amyloid plaque accumulation in the initial deposition period
mainly at the hippocampus/neocortex, consistent with previous reported studies
[30,31], whereas at later stages sparse Aβ-fibrillar plaques are progressively
observed also at nearby cortical areas. After the preventive immunization
period with EB101 vaccine, a significant reduction in density of Aβ plaque
burden was observed in APP/PS1 transgenic mouse regions, as well as reduced
burden areas when compared with APP/PS1 mice treated with different EB101
vaccine components or PBS. Schenk and colleagues [4] reported similar
preventive immunization results in PDAPP mice, although differences in
immunization efficiency and vaccine conformation have been improved in the present
study. During this preventive period, EB101 immunization also prevented the
progressive development of other AD pathological effects such as dystrophic
plaque neurites, astrocytosis and motor coordination deficits. The hindrance of
massive glial activation in response to early development of Aβ deposits has
been accepted by the scientific community as the main key in efficient
preventive immunization [32-35], this being the case of our EB101 vaccine,
which has demonstrated, when compared with different EB101 vaccine components
or PBS-treated mouse groups, that it prevents the development of astrocyte
activation. Since the activation of astrocytes and microglia has been reported
to induce the degradation of Aβ [36,37], the near-absence of Aβ-related astrocytosis
in cortex and hippocampus of mice treated with EB101 vaccine confirmed the
preventive effect against the development of AD-like pathology in this mouse
model.
ACKNOWLEDGEMENTS
This work was supported by the III Research Grant Program of the Camilo
José Cela University 2014-15 (TransBioModel – 2014/36).
- Tanzi R, Bertram L (2005)
Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic
perspective. Cell 120:
545-555.
- Blennow K, De Leon M,
Zetterberg H (2006) Alzheimer’s disease. Lancet 368: 387-403.
- Masliah E, Hansen L, Adame A,
et al. (2005) Abeta vaccination effects on plaque pathology in the absence
of encephalitis in Alzheimer disease. Neurology 64:129-131.
- Schenk D, Barbour R, Dunn W,
et al. (1999) Immunization with amyloid-beta attenuates
Alzheimer-disease-like pathology in the PDAPP mouse. Nature 400:173-177.
- Carrera I, Etcheverria I,
Fernandez-Novoa L, et al. (2012) Vaccine development to treat Alzheimer´s
disease neuropathology in APP/PS1 transgenic mice. Int J Alzheimers Dis
376138.
- Carrera I, Etcheverría I, Li
Y, et al. (2013) Immunocytochemical Characterization of Alzheimer Disease
Hallmarks in APP/PS1 Transgenic Mice Treated with a New Anti-Amyloid- β
Vaccine. Biomed Res Int 709145.
- Davtyan H, Bacon A,
Petrushina I, et al. (2014) Immunogenicity of DNA- and recombinant
protein-based Alzheimer Disease epitope vaccines. Hum Vaccin Immunother
13:10.
- Rafii MS (2013) Update on
Alzheimer's disease therapeutics. Rev Recent Clin Trials 2: 110-118.
- Nicoll J, Wilkinson D,
Holmes, et al. (2003) Neuropathology of human Alzheimer disease after
immunization with amyloid-beta peptide: a case report. Nat Med 9: 448-452.
- Ferrer I, Boada M,
Sánchez-Guerra, et al. (2004) Neuropathology and pathogenesis of
encephalitis following amyloid-beta immunization in Alzheimer’s disease.
Brain Pathol 14: 11-20.
- Blaho VA, Hla T (2014) An
update on the biology of sphingosine 1-phosphate receptors. J Lipid Res
55: 1596-1608
- Nishi T,
Kobayashi N, Hisano Y, et al. (2014) Molecular and physiological functions of
sphingosine 1-phosphate transporters. Biochim Biophys Acta 5: 759-765.
- Kajimoto T, Okada T, Yu H, et
al. (2007) Involvement of sphingosine-1-phosphate in glutamate secretion
in hippocampal neurons. Mol Cell Biol 9: 3429-40.
- Gräler MH (2012) The role of
sphingosine 1-phosphate in immunity and sepsis. Am J Clin Exp Immunol 2:
90-100.
- Singh IN, Hall ED (2008)
Multifaceted roles of sphingosine-1-phosphate: how does this bioactive
sphingolipid fit with acute neurological injury? J Neurosci Res 7:
1419-1433.
- Hopson KP, Truelove J, Chun
J, et al. (2011) S1P activates store-operated calcium entry via receptor-
and non-receptor-mediated pathways in vascular smooth muscle cells. Am J
Phy Cell Physiol 4: 919-26.
- Ratajczak MZ, Suszynska M,
Borkowska S, et al. (2014) The role of sphingosine-1 phosphate and
ceramide-1 phosphate in trafficking of normal stem cells and cancer cells.
Expert Opin Ther Targets 1: 95-107.
- Zhang YH, Vasko MR, Nicol GD
(2006) Intracellular sphingosine 1-phosphate mediates the increased
excitability produced by nerve growth factor in rat sensory neurons. J
Physiol 1: 101-13.
- Kays JS, Li C, Nicol GD
(2012) Expression of sphingosine 1-phosphate receptors in the rat dorsal
root ganglia and defined single isolated sensory neurons. Physiol Genomics
18: 889-901.
- Kimura A, Ohmori T,
Kashiwakura Y, et al. (2008) Antagonism of sphingosine 1-phosphate
receptor-2 enhances migration of neural progenitor cells toward an area of
brain. Stroke 12: 3411-7.
- Biswas K, Yoshioka K, Asanuma
K, et al. (2013) Essential role of class II
phosphatidylinositol-3-kinase-C2α in sphingosine 1-phosphate
receptor-1-mediated signaling and migration in endothelial cells. J Biol
Chem 4: 2325-39.
- Davtyan H, Petrushina I,
Ghochikyan A (2014) Immunotherapy for Alzheimer's Disease: DNA- and
Protein-Based Epitope Vaccines. Methods Mol Biol 1143: 259-81.
- Wisniewski T, Goñi F (2014)
Immunotherapy for Alzheimer's disease. BiochemPharmacol 4: 499-507.
- Schenk D, Barbour R, Dunn W,
et al. (1999) Immunization with amyloid-β attenuates Alzheimer
disease-like pathology in the PDAPP mouse. Nature 400: 173-177.
- Wilcock DM, Gharkholonarehe
N, Van Nostrand WE, et al. (2009) Amyloid reduction by amyloid-β
vaccination also reduces mouse tau pathology and protects from neuron loss
in two mouse models of Alzheimer’s disease. J Neurosci 29: 7957-7965.
- Ghochikyan A, Mkrtichyan M,
Petrushina I, et al. (2006) Prototype Alzheimer's disease epitope vaccine induced
strong Th2-type anti-Abeta antibody response with Alum to Quil A adjuvant
switch. Vaccine 13: 2275-82.
- Panza F,
Frisardi V, Solfrizzi V, et al. (2012) Immunotherapy for Alzheimer's disease:
from anti-β-amyloid to tau-based immunization strategies. Immunotherapy 2:
213-38.
- Mount A, Koernig S, Silva A,
et al. (2013) Combination of adjuvants: the future of vaccine design.
Expert Rev Vaccines 7: 733-46.
- Lannfelt L, Relkin NR,
Siemers ER (2014) Amyloid-ß-directed immunotherapy for Alzheimer's
disease. J Intern Med 3: 284-95.
- Trinchese F, Liu S, Battaglia
F, et al. (2004) Progressive age-related development of Alzheimer-like
pathology in APP/PS1 mice. Ann Neurol 55: 801-814.
- Jankowsky J, Fadale D,
Anderson et al. (2003) Mutant presenilins specifically elevate the levels
of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation
of a 42-specific gamma secretase. Hum Mol Genet 13: 159-170.
- Davtyan H, Ghochikyan A,
Petrushina I, et al. (2013) Immunogenicity, efficacy, safety, and
mechanism of action of epitope vaccine (Lu AF20513) for Alzheimer's
disease: prelude to a clinical trial. J Neurosci 11: 4923-34.
- Zotova E, Holmes C, Johnston
D, et al. (2011) Microglial alterations in human Alzheimer's disease
following Aβ42 immunization. Neuropathol Appl Neurobiol 5: 513-24.
- Cacabelos R, Martínez R.,
Fernández-Novoa L, et al. (2012) Genomics of Dementia: APOE- and
CYP2D6-Related Pharmacogenetics. Int J Alzheimers Dis 518901.
- Cacabelos R, Martínez-Bouza R
(2011) Genomics and pharmacogenomics of dementia. CNS Neurosci Ther 17:
566-76.
- Morales I,
Guzmán-Martínez L, Cerda-Troncoso C, et al. (2014) Neuroinflammation in the
pathogenesis of Alzheimer's disease. A rational framework for the search
of novel therapeutic approaches. Front Cell Neurosci 8: 112.
- Birch AM, Katsouri L, Sastre
M (2014) Modulation of inflammation in transgenic models of Alzheimer's
disease. J Neuroinflammation 11: 25.
- Schroeter S, Khan K, Barbour
R, et al. (2008) Immunotherapy reduces vascular amyloid-beta in PDAPP
mice. J Neurosci 28: 6787-6793.
- Suo Z, Cox
A, Bartelli N, et al. (2007) GRK5 deficiency leads to early Alzheimer-like pathology and
working memory impairment. Neurobiol Aging 28: 1873-1888.
- Morgan D (2006) Modulation of
microglial activation state following passive immunization in amyloid
depositing transgenic mice. Neurochem Int 49: 190-194.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Microbiology and Microbial Infections (ISSN: 2689-7660)
- Food and Nutrition-Current Research (ISSN:2638-1095)
- Journal of Genetics and Cell Biology (ISSN:2639-3360)
- Journal of Womens Health and Safety Research (ISSN:2577-1388)
- Proteomics and Bioinformatics (ISSN:2641-7561)
- Journal of Astronomy and Space Research
- Advances in Nanomedicine and Nanotechnology Research (ISSN: 2688-5476)